Abstract

Introduction Primary central nervous system lymphoma (PCNSL) is a rare B cell lymphoma with an aggressive course. Relapses remain common even with intensive immuno-chemotherapy treatment options such as MATRix and consolidation with autologous stem cell transplant (ASCT), and prognostic tools are limited. Although, double expressor status (co-expression of both MYC and BCL-2) is well established in systemic de novo DLBCL, its prognostic impact in PCNSL has been understudied. Here we evaluated the prognostic value of BCL-2, BCL-6, and MYC expression in a retrospective cohort of treated PCNSL patients diagnosed at 7 academic centres in the UK.
Methods We performed this retrospective analysis on histologically-confirmed PCNSL patients diagnosed consecutively between 1st May 2015 and 31st May 2020. Treatment with a high-dose methotrexate-based chemotherapy regimen was a mandatory inclusion criterion. BCL-2 and MYC immunohistochemistry (IHC) protein expression data were collated from local diagnostic histology reports and double expressor (DE) status was defined as per WHO criteria (MYC: surface expression > 40% of cells and BCL-2: >50% of cells). Outcomes included response rates, progression-free survival (PFS) and overall survival (OS). Where available, diagnostic fluorescence in situ hybridisation (FISH) data was collected for BCL-2, BCL-6 and MYC loci translocations. Cox-regression for PFS and OS were used to determine baseline predictors of response and survival and to construct multivariable models for factors associated with outcome.
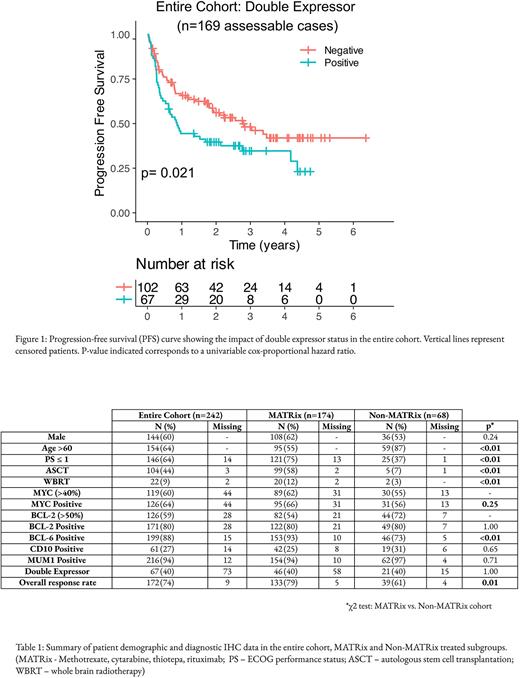
Results In total, 242 patients were analysed. Key baseline patient characteristics and IHC data are summarised in Table 1. Median age was 65 years (IQR 56-71), 60% were male and 64% of patients had an ECOG performance status (PS) of 0-1. One hundred and seventy-four (72%) patients received treatment with MATRix chemotherapy; these patients were younger (median age 62 vs. 73 years, p< 0.01), had better baseline PS (PS ≤ 1: 75% vs. 37%, p< 0.01) and higher rates of consolidation with BCNU-thiotepa ASCT (58% vs. 7%, p< 0.01).
Double expressor (DE) status was assessable in 169/242 (69%) patients. Reasons for absent DE status were missing IHC expression data (BCL-2: n=28; MYC: n=44) or IHC expression data not reported in line with WHO recommended criteria (BCL-2: n=45; MYC: n=7). In the DE-assessable cohort (n = 169 patients), 40% of patients had DE PCNSL, higher than estimates of DE prevalence in systemic DLBCL (reported rates between 20 and 30%). As only 40 cases (17%) underwent FISH assessment for concurrent MYC and BCL-2 loci translocation (double hit status), correlation between double hit and DE status was not possible. DE status was not associated with patient age at diagnosis, baseline PS or treatment received. With a median follow up of 1.7 years, 34% of patients relapsed and 45% died. Although single MYC or BCL-2 expression alone had no significant association with OS or PFS in the entire cohort, DE status was associated with significantly poorer PFS, and earlier relapse (median 0.86 years vs. 2.77 years; HR 1.61 (1.07 - 2.41); p=0.021) (Fig. 1). In a subgroup analysis restricted to patients treated with MATRix chemotherapy (n = 116 patients), there was a trend towards inferior PFS and OS, although this did not reach significance in a univariable analysis. However, the adverse prognostic impact of DE status on PFS was maintained in a multivariable analysis modelling age, baseline PS, treatment received, ASCT consolidation status and DE status both for the entire cohort (HR 1.75 (1.15 - 2.66); p<0.01) and the MATRix treated subgroup (HR 1.91 (1.08 - 3.36); p=0.03). A trend towards an association between DE status and OS was noted but did not reach significance in either a univariable or multivariable analysis.
We observed no significant difference in PFS or OS between DE-PCNSL and non-DE-PCNSL patients who received MATRix and consolidation with ASCT, perhaps indicating that the adverse impact of DE status is modified by this treatment strategy, although the sample size was small (n=66).
Conclusions In a large, retrospective cohort of PCNSL patients treated at 7 UK academic treatment centres, double expression of MYC and BCL-2 by IHC was associated with inferior PFS even in patients treated with MATRix chemotherapy. Validation of this finding in independent cohorts is warranted.
___
JO, CPF and KC share senior authorship
Disclosures
Marafioti:UCL-BRC: Research Funding. Chaganti:AbbVie: Consultancy, Honoraria; Adicet Bio: Consultancy, Honoraria; Atara Biotherapeutics: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Gilead/Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Orion Pharma: Consultancy, Honoraria; Pierre Fabre: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. McKay:Gilead/Kite, Incyte, Janssen: Speakers Bureau; Abbvie, AstraZeneca, Beigene, Celgene/BMS, Epizyme, Gilead/Kite, Incyte, Janssen, Recordati Rare Diseases, Roche, Takeda: Consultancy. Smith:Abbvie, AstraZeneca, Janssen: Consultancy; Abbvie, AstraZeneca: Speakers Bureau; AstraZeneca: Other: Travel to scientific congress. Eyre:Loxo Oncology @ Lilly: Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView: Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Medscape: Speakers Bureau. Martinez-Calle:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Other: travel support; Abbvie: Consultancy, Honoraria, Other: travel support; Janssen: Honoraria. Cwynarski:BeiGene: Research Funding; Roche, Celgene/BMS, Takeda, KITE: Other: Travel to scientific congress; Roche, Takeda, KITE/Gilead, Incyte: Speakers Bureau; Roche, Takeda, Celgene/BMS, Atara, Gilead/KITE, Janssen, Incyte: Consultancy. Fox:Roche: Other: Travel to scientific congress; BeiGene: Research Funding; Celgene/BMS, Gilead/Kite, Incyte, Janssen, Roche, Takeda: Speakers Bureau; Abbvie, AstraZeneca, Atarabio, Celgene/BMS, GenMab, Gilead/Kite, Incyte, Janssen, Morphosys, Ono, Roche, Takeda: Consultancy. Okosun:Gilead: Honoraria, Research Funding; Eisai: Honoraria; AstraZeneca: Honoraria; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.